35 Br Bromine 79.90 |
Bromine is a halogen that is liquid at room temperature.
Bromine can be added to an aromatic ring via EAS. This reaction is catalyzed by a lewis acid such as Irontribromide, or Aluminumtribromide.
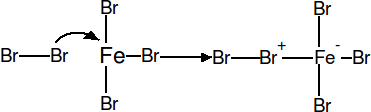
In the first step of the reaction, Bromine coordinates to the Irontribromide catalyst. This weakens the Br - Br bond, thus making the species a better electrophile.
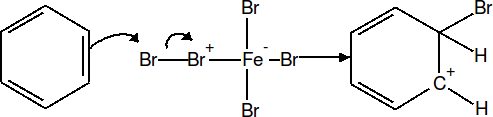
In the second step of the reaction, the electron rich pi cloud of the benzene ring attacks the nucleophile generated in step 1. This intermediate is very unstable because the aromaticity of the ring has been disrupted.
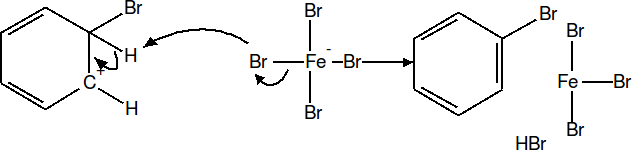
Finally, the Hydrogen is removed from the Carbon that was attacked in step 2, and the electrons dump back into the pi cloud restoring the aromaticity of the ring.